[Literature Insight] From CRISPR Screening to FCD Mechanisms: Do Cilia Also Influence Nerves?
![]()
Cilia protrude from the surface of most cells in the body and play a crucial role in cellular signaling. Research has shown that defects in ciliary assembly can lead to "ciliopathies" in humans, characterized by multisystem developmental disorders. Additionally, cilia are typically resorbed prior to cell growth and differentiation to maintain normal cellular function. However, our understanding of the regulatory mechanisms underlying the critical process of regulated ciliary disassembly remains limited.
On October 29, 2025, David K. Breslow's team at Yale University published a research paper titled "A CRISPR activation screen reveals a cilia disassembly pathway mutated in focal cortical dysplasia" in Science Advances. This study employed a genome-wide CRISPR library screening strategy, utilizing the CRISPRa system to construct an sgRNA library targeting 22,774 genes, to systematically identify regulators that promote ciliary disassembly.
On October 29, 2025, David K. Breslow's team at Yale University published a research paper titled "A CRISPR activation screen reveals a cilia disassembly pathway mutated in focal cortical dysplasia" in Science Advances. This study employed a genome-wide CRISPR library screening strategy, utilizing the CRISPRa system to construct an sgRNA library targeting 22,774 genes, to systematically identify regulators that promote ciliary disassembly.
Original link: https://www.science.org/doi/10.1126/sciadv.aeb7238
Spotlight
1. Innovative Screening Strategy: CRISPRa Technology Uncovers Negative Regulators of Cilia
The research team established a mouse NIH-3T3 ciliary cell line expressing dCas9-VP64 and conducted a genome-wide CRISPRa screen using a Blasticidin selection reporter system dependent on the Hedgehog (Hh) signaling pathway. By overexpressing 22,774 mouse genes, key ciliary disassembly regulators such as F2R and SARM1 were identified.
2. Mechanistic Breakthrough: Discovery of a SARM1-Mediated Calcium-Dependent Ciliary Disassembly Pathway
The study revealed a SARM1-mediated calcium-dependent ciliary disassembly pathway: thrombin activates the receptor F2R, leading to the production of the second messenger cADPR by SARM1, which activates RyR to induce calcium release from the endoplasmic reticulum. The localized increase in calcium subsequently triggers the RhoA/ROCK pathway, driving ciliary disassembly.
3. Disease Relevance: Gene Mutations in the Ciliary Disassembly Pathway Linked to Various FCD Subtypes
The study found that mutations in genes such as SARM1, RYR2/3, and RHOA in patients with Focal Cortical Dysplasia (FCD) lead to excessive ciliary disassembly and impaired cortical neuron development. Although the pathogenic genes differ across FCD subtypes, they all rely on the SARM1 pathway to mediate ciliary disassembly, highlighting its role as a common key mechanism in FCD pathogenesis.
01
Identification and Validation of F2R and SARM1
in Inducing Ciliary Disassembly
in Inducing Ciliary Disassembly
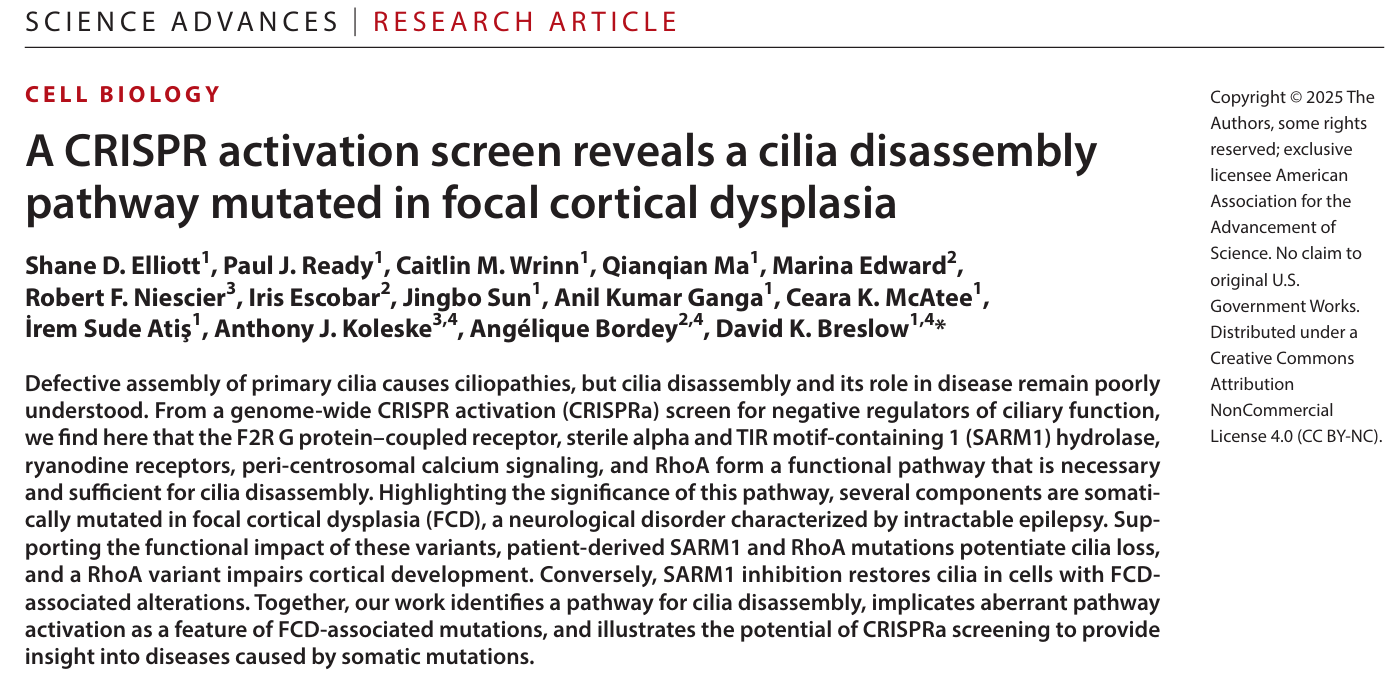
Through a genome-wide CRISPRa screen (covering 22,774 genes), the research team identified F2R and SARM1 as key regulators of ciliary disassembly. Functional validation demonstrated that overexpression of either gene significantly reduced cilia, while their specific inhibitors (vorapaxar and DSRM-3716) completely rescued ciliary loss.
Subsequently, the team conducted doxycycline (Dox)-induced F2R overexpression experiments, confirming that F2R can trigger disassembly of pre-existing cilia.
Subsequently, the team conducted doxycycline (Dox)-induced F2R overexpression experiments, confirming that F2R can trigger disassembly of pre-existing cilia.
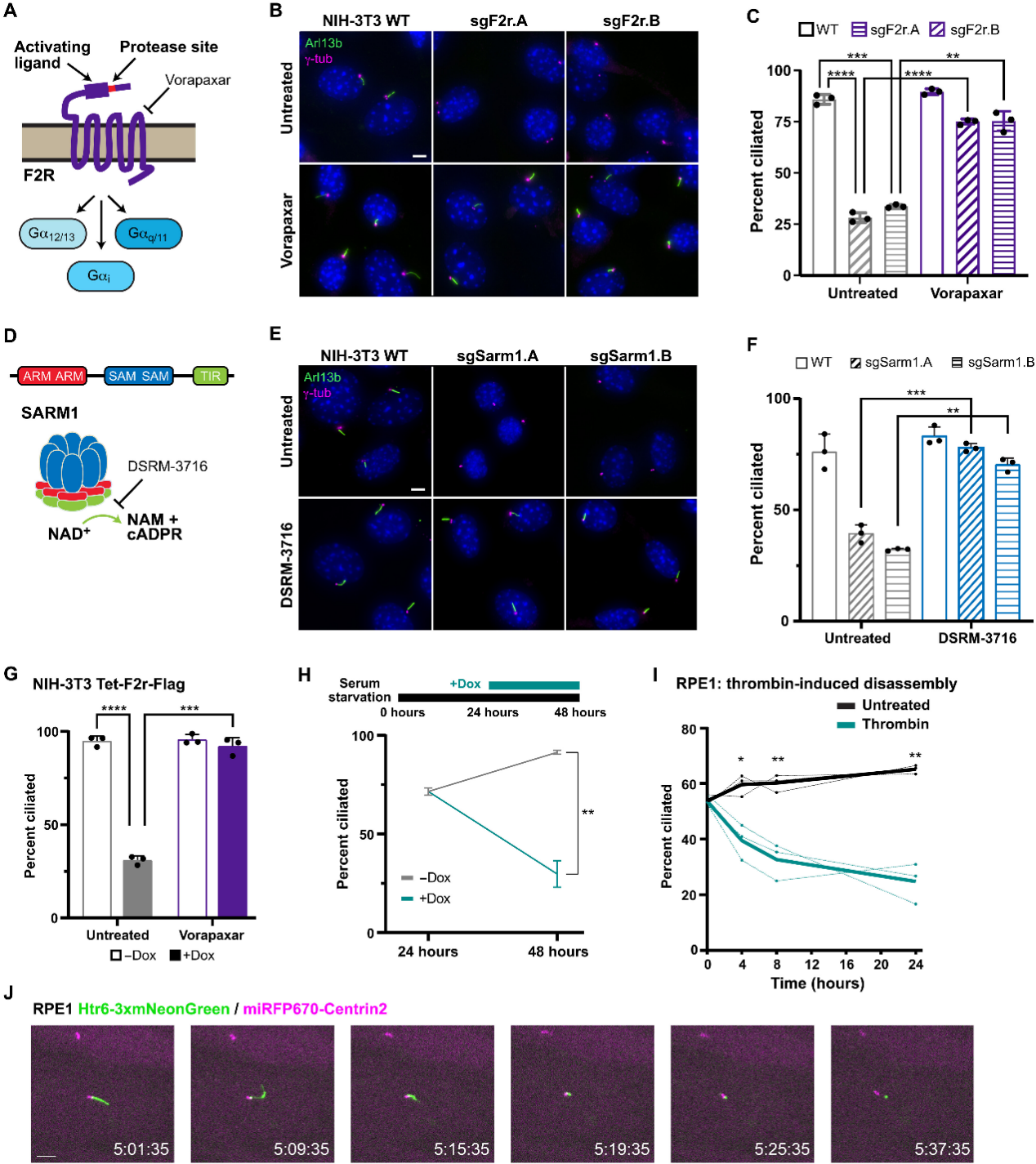
Figure 1. Overexpression of F2R or SARM1 Induces Ciliary Disassembly
02
Elucidation of Ciliary Disassembly Mechanisms
and Subcellular Localization
and Subcellular Localization
The study further delineated the mechanism and subcellular localization of F2R–SARM1-mediated ciliary disassembly.
Experiments revealed that SARM1 acts downstream of F2R activation. Moreover, using inhibitors such as dantrolene (an RyR inhibitor) and the calcium chelator BAPTA-AM, it was found that cADPR produced by SARM1 activates RyR channels, leading to calcium release. This subsequently triggers ciliary disassembly via RhoA-targeted activation of ROCK kinase.
Experiments revealed that SARM1 acts downstream of F2R activation. Moreover, using inhibitors such as dantrolene (an RyR inhibitor) and the calcium chelator BAPTA-AM, it was found that cADPR produced by SARM1 activates RyR channels, leading to calcium release. This subsequently triggers ciliary disassembly via RhoA-targeted activation of ROCK kinase.
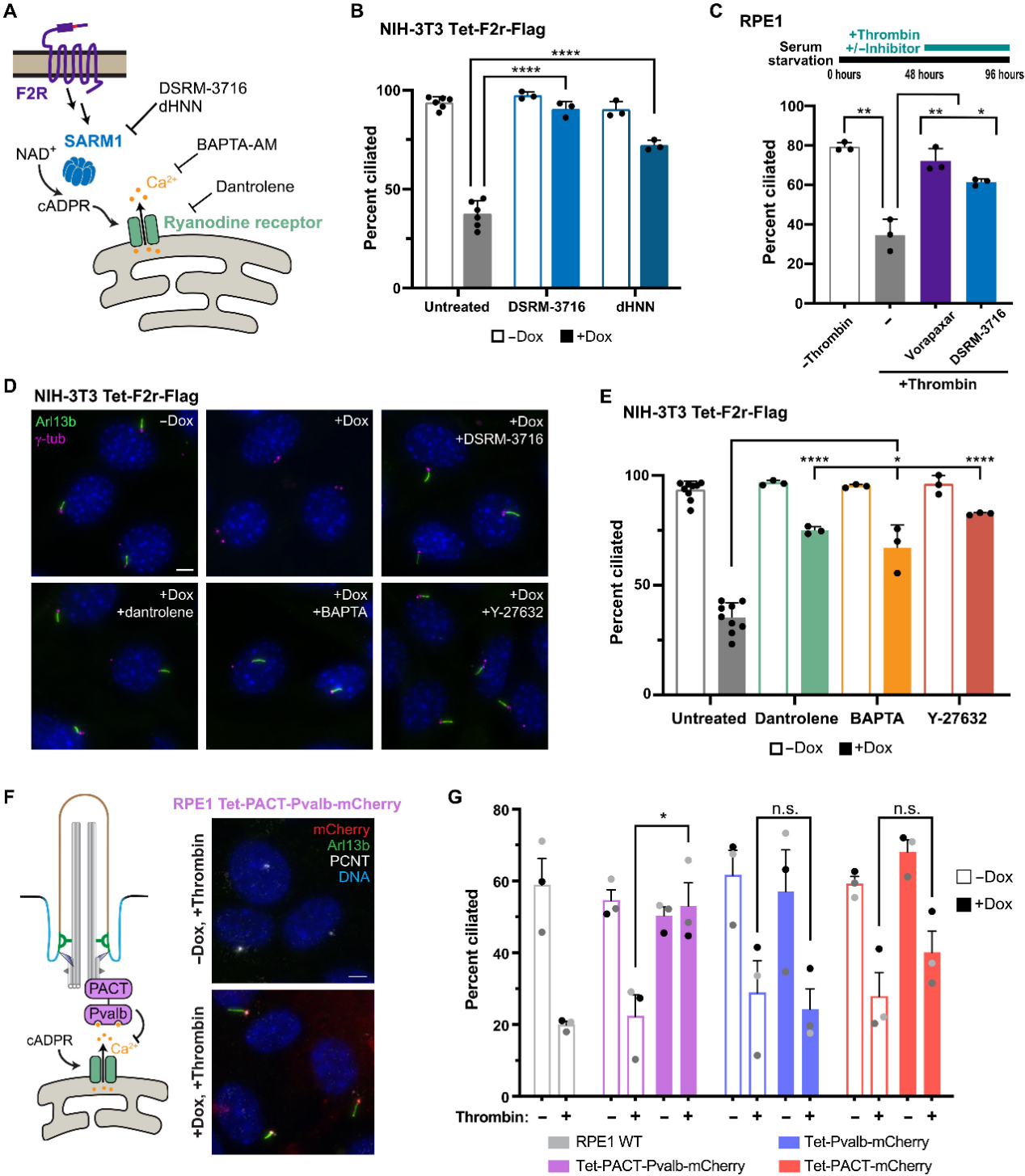
Figure 2. SARM1-Triggered Signaling Pathway Mediates Ciliary Disassembly
03
Validation of Endogenous Regulation
During serum- or lysophosphatidic acid (LPA)-induced ciliary disassembly, inhibition of SARM1, RyR, or ROCK significantly preserved ciliary stability. This confirms that the F2R–SARM1–RyR–RhoA/ROCK pathway is an endogenous key pathway mediating serum mitogen-induced ciliary disassembly.
Figure 3. Endogenous Regulation of Ciliary Dynamics by the SARM1-Driven Pathway
04
Therapeutic Potential of Targeting the Pathway in FCD
The study revealed that FCD patients carry mutations in genes such as SARM1, RYR2/3, and RHOA, which overlap with this ciliary disassembly pathway. These mutations were found to cause abnormal development of cortical neurons.
Subsequently, the research team constructed disease models including SARM1-G528S, RhoA-P75S, and mTORC1-activating mutants to simulate the pathogenic mechanisms of different FCD subtypes. The results demonstrated that inhibiting the SARM1/RyR/RhoA pathway exhibited therapeutic efficacy against both Type I and Type II FCD.
Subsequently, the research team constructed disease models including SARM1-G528S, RhoA-P75S, and mTORC1-activating mutants to simulate the pathogenic mechanisms of different FCD subtypes. The results demonstrated that inhibiting the SARM1/RyR/RhoA pathway exhibited therapeutic efficacy against both Type I and Type II FCD.
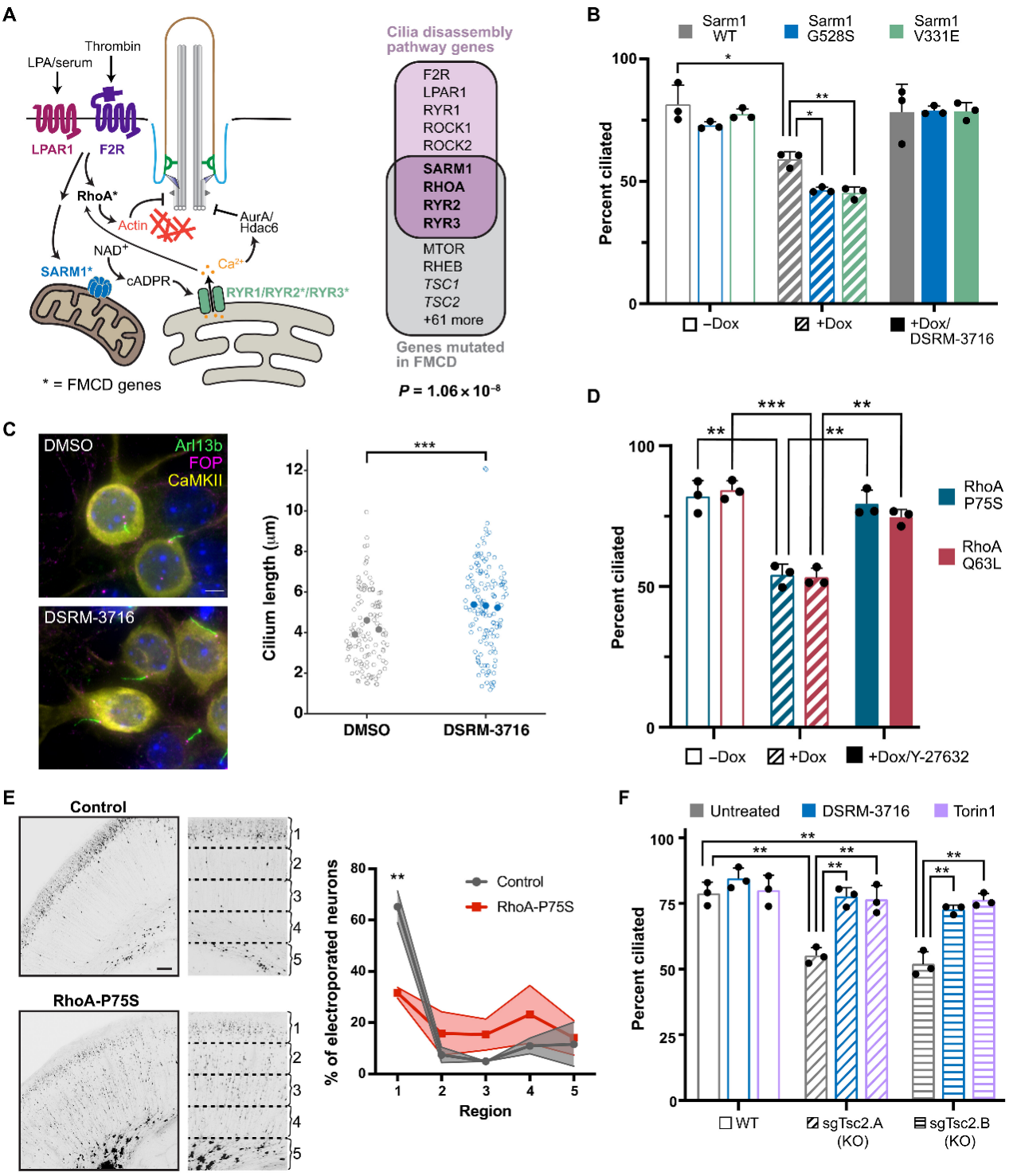
Figure 4. Components of the Ciliary Disassembly Pathway Are Mutated in FCD
05
Summary
This study, through CRISPRa screening, identified a complete ciliary disassembly signaling pathway: F2R → SARM1 → cADPR → RyR → calcium ions → RhoA. More importantly, it establishes the first link between abnormalities in the ciliary disassembly pathway and the pathogenesis of Focal Cortical Dysplasia (FCD), highlighting the therapeutic potential of targeting this pathway in FCD.
Future research should aim to further elucidate the connection mechanism between F2R/LPAR1 and SARM1, delineate the molecular details of how RhoA/ROCK drives ciliary disassembly, and validate the prevalence and pathological contribution of ciliary defects in FCD patient tissues.
Future research should aim to further elucidate the connection mechanism between F2R/LPAR1 and SARM1, delineate the molecular details of how RhoA/ROCK drives ciliary disassembly, and validate the prevalence and pathological contribution of ciliary defects in FCD patient tissues.
EDITGENE offers comprehensive end-to-end solutions for CRISPR library screening, from custom sgRNA library design and stable Cas9 cell line development to lentiviral library packaging, library cell pool establishment, functional screening experiments, and NGS data analysis. We provide a wide selection of popular pre-designed libraries and most extensive collection of ready-to-ship library plasmids and library viruses —all available within one week. Place your order and start screening immediately!

















