[Literature Review] Could Lipids Released from Tumor Necrosis Undermine Immune Cell Function?
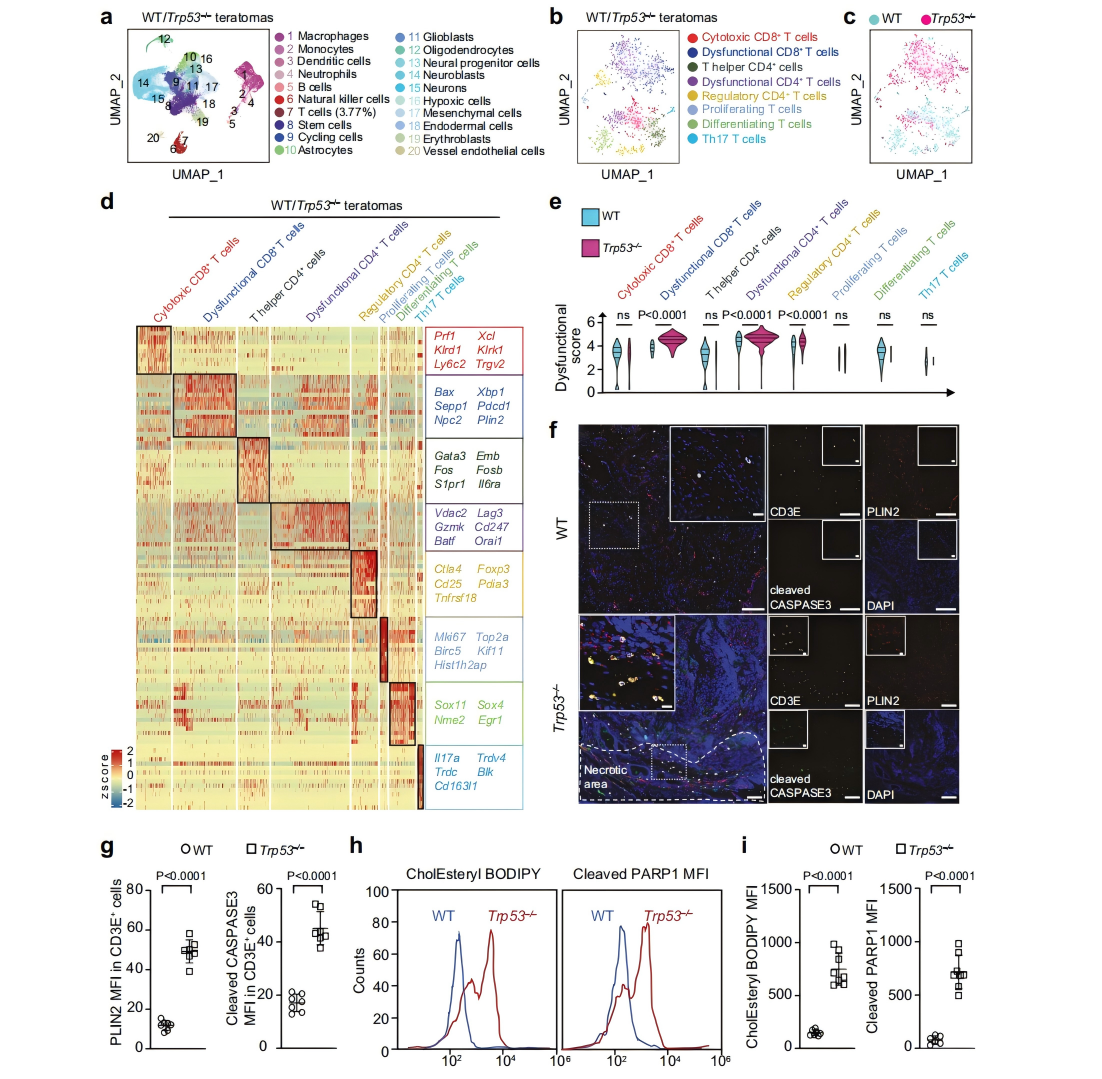
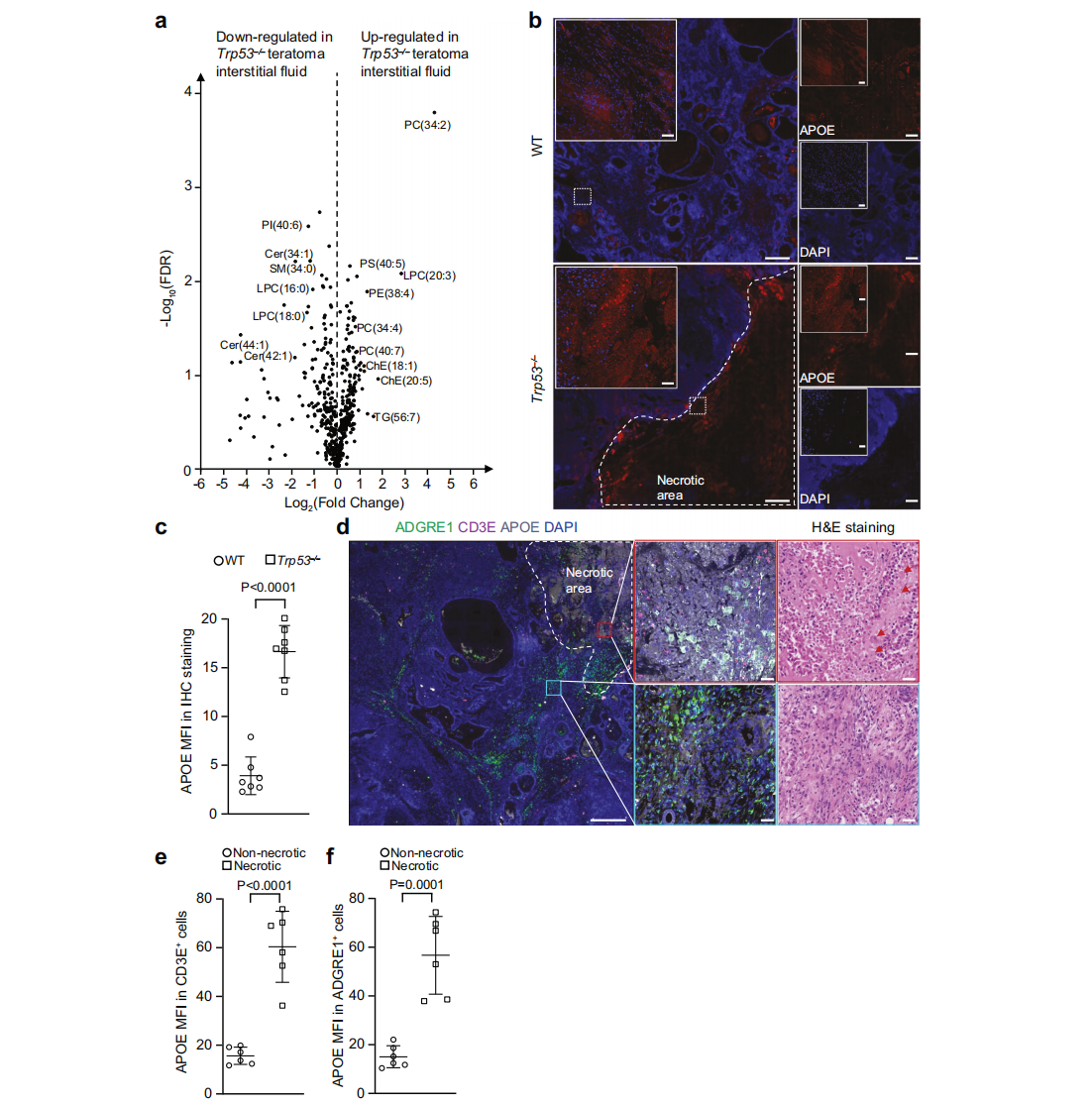
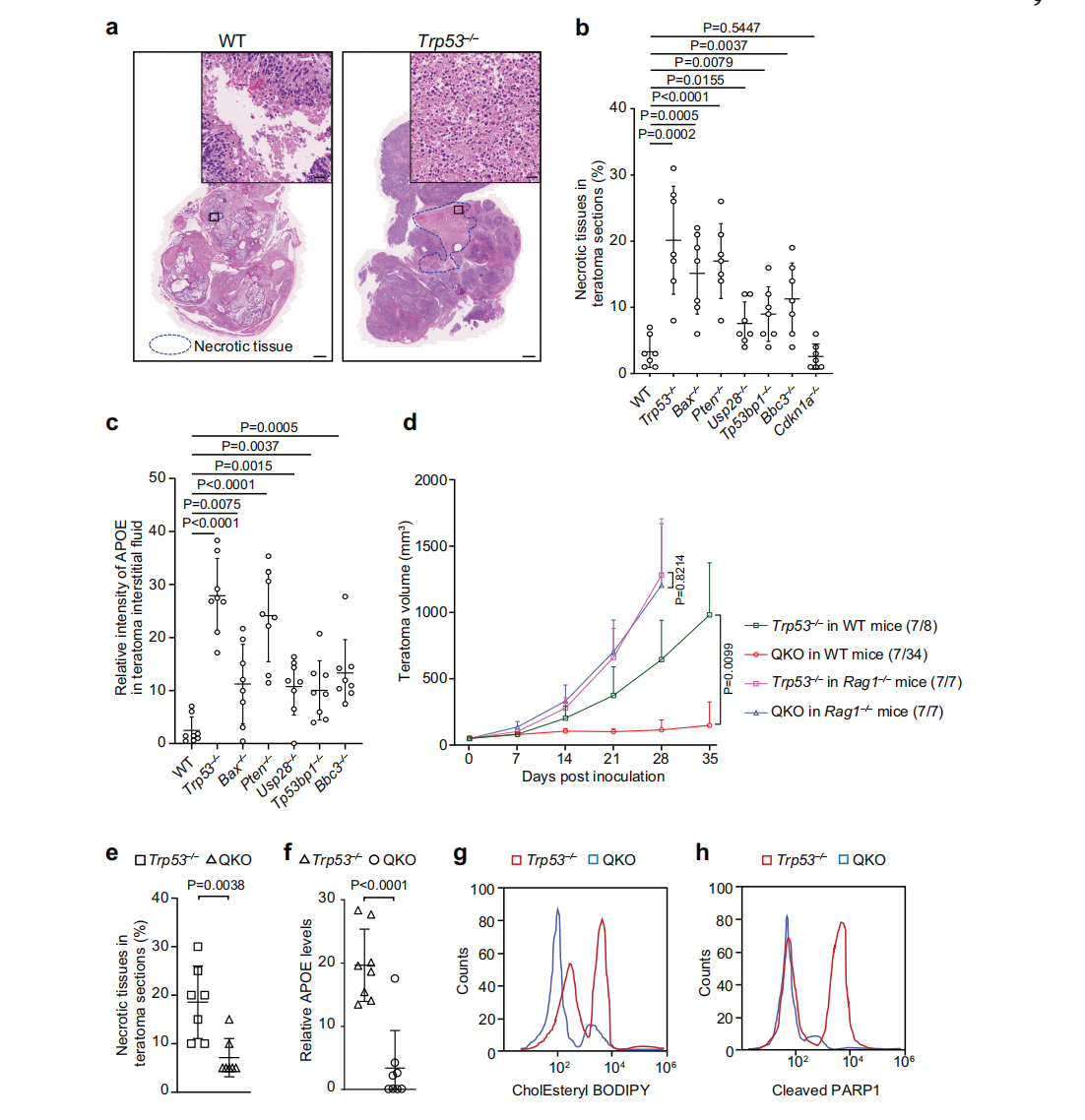
Immune evasion is a hallmark of cancer, and glioblastoma (GBM) has so far shown poor responses to currently available immune checkpoint inhibitors (ICIs). In September 2025, a collaborative study by Westlake University and the University of Texas Southwestern Medical Center reported their findings in Cell Research under the title “Targeting necrotic lipid release in tumors enhances immunosurveillance and cancer immunotherapy of Glioblastoma.” In this work, the researchers used non-cancerous mouse embryonic stem cells to establish a syngeneic teratoma model. Through CRISPR screening in this model, they successfully identified key genes that determine tumor immune evasion. Original link: https://doi.org/10.1038/s41422-025-01155-y Spotlight 1. Innovative model construction: This study is the first to establish an isogenic teratoma model using non-cancerous mouse embryonic stem cells, providing a precise platform for investigating early mechanisms of immune evasion. 2. Identification of key genes: The researchers introduced a genome-wide CRISPR library into C57BL/6J mouse embryonic stem cells to generate a large-scale knockout cell pool. These cells were then either maintained in vitro or transplanted into wild-type and Rag1⁻/⁻ immunodeficient mice to form teratomas. Enrichment analysis revealed that sgRNAs targeting Trp53 and Pten were specifically enriched in teratomas formed in immunocompetent mice, but not in immunodeficient hosts. Follow-up studies using knockout cell lines confirmed that Trp53⁻/⁻ and Pten⁻/⁻ cells exhibited significantly enhanced tumorigenic capacity in wild-type mice, indicating that the loss of these genes facilitates tumor immune evasion. Figure 1. Genome-wide CRISPR screening of immune surveillance genes Single-cell RNA sequencing (scRNA-seq) lineage analysis revealed that Trp53⁻/⁻ teratomas exhibit abnormal immune infiltration, characterized by marked enrichment and dysfunction of CD4⁺ T cells, CD8⁺ T cells, and regulatory T cells. Figure 2. Molecular characterization of infiltrating T cells in teratomas by single-cell RNA sequencing Lipidomic analysis revealed elevated levels of polyunsaturated phospholipids, cholesterol esters, and other lipids in the stromal fluid of Trp53⁻/⁻ teratomas. Enhanced extracellular APOE deposition was observed in necrotic regions, accompanied by significant accumulation of APOE within infiltrating immune cells. The study demonstrated that Trp53 loss promotes necrosis, thereby increasing APOE release. These released APOE particles are subsequently taken up by immune cells, leading to their functional impairment. Figure 3. Necrotic teratoma cells release APOE lipid particles into the tumor microenvironment The study found that cell necrosis is primarily dependent on the mitochondrial permeability transition pore (mPTP) pathway. The researchers generated mPTP-deficient knockout cell lines and observed a marked reduction in cell necrosis, leading to decreased APOE release. As a result of reduced APOE release, lipid accumulation within T cells was significantly diminished, ultimately restoring immune surveillance in vivo. Figure 4. Blocking necrosis eliminates immune evasion in Trp53⁻/⁻ teratomas This study reveals an APOE lipid particle-mediated immune evasion mechanism in TP53-mutant glioblastoma, leading to dysfunction of immune cells. In the future, targeted interventions against APOE lipid particles, particularly in combination with immune checkpoint inhibition, may offer a potential therapeutic strategy for TP53-mutant glioblastoma. These findings also provide a reference framework for investigating similar mechanisms in other tumor types.

Genome-wide CRISPR screening revealed that immune evasion is closely associated with the loss of pro-apoptotic tumor suppressor genes such as Trp53 and Pten.
3. Discovery of novel therapeutic targets:
The study uncovered the central role of APOE lipid particles in tumor immune evasion, offering new strategies for glioblastoma treatment.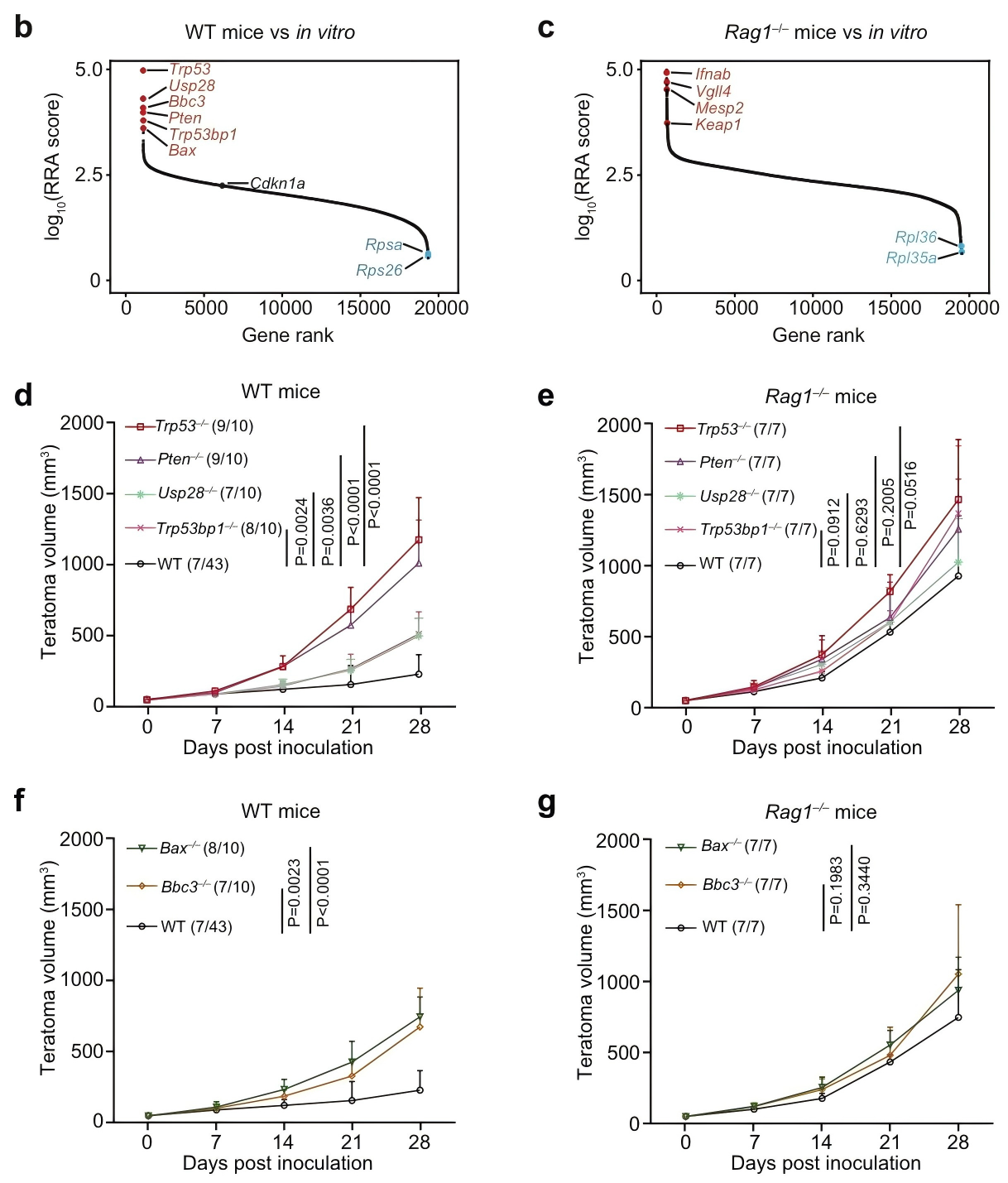
These T-cell populations displayed elevated expression of exhaustion- and apoptosis-associated genes, as well as lipid metabolism–related genes. Follow-up experiments confirmed that within Trp53⁻/⁻ tumors, T cells showed significant increases in lipid droplet accumulation, cholesterol ester levels, and apoptotic markers. Meanwhile, tumor-associated macrophages (TAMs), another key immune cell population in the tumor microenvironment, exhibited similar lipid deposition and apoptotic features.