[Literature Review] Bone Marrow–Targeted LNPs Open New Paths for Hematological Treatment
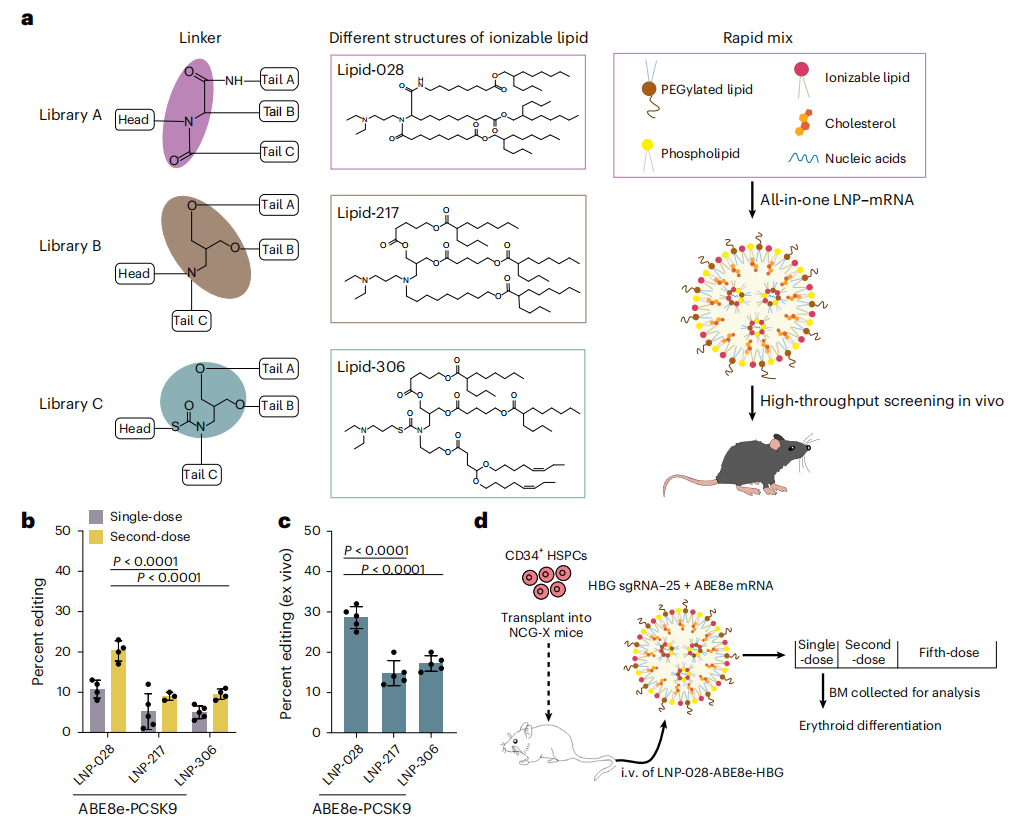
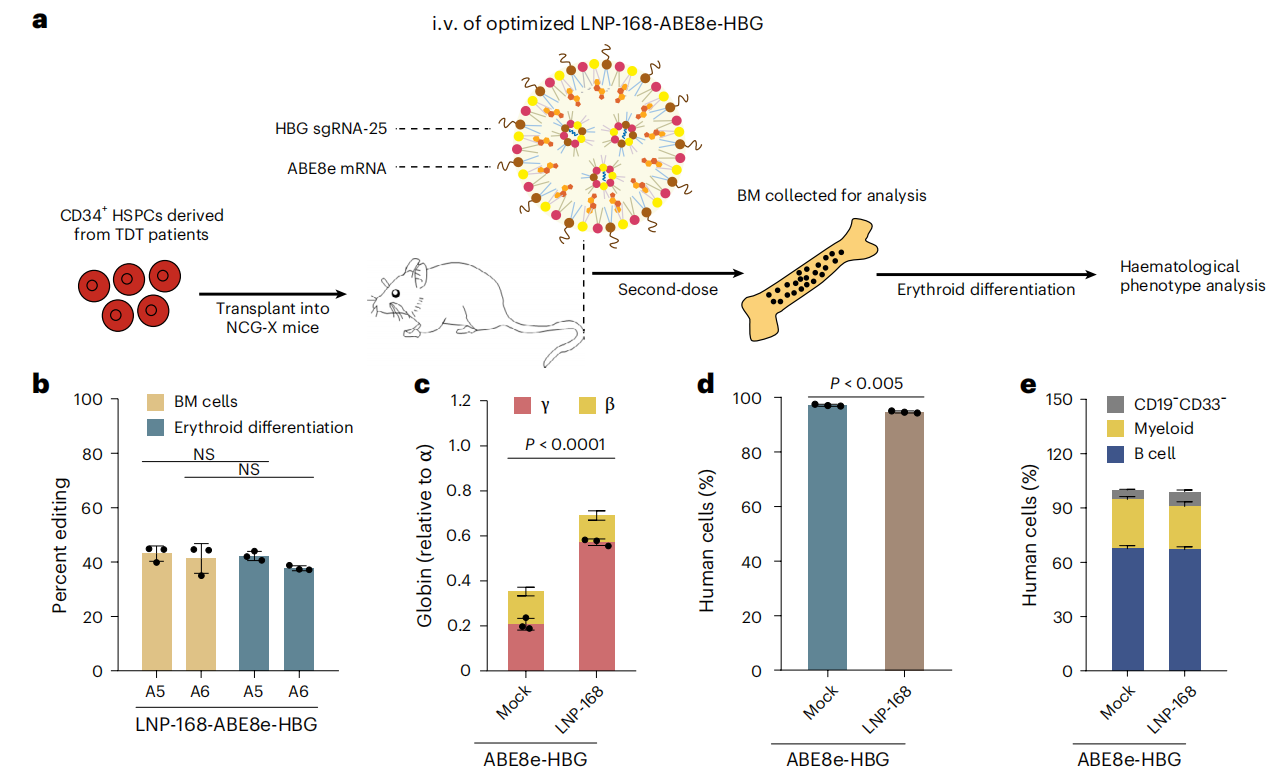
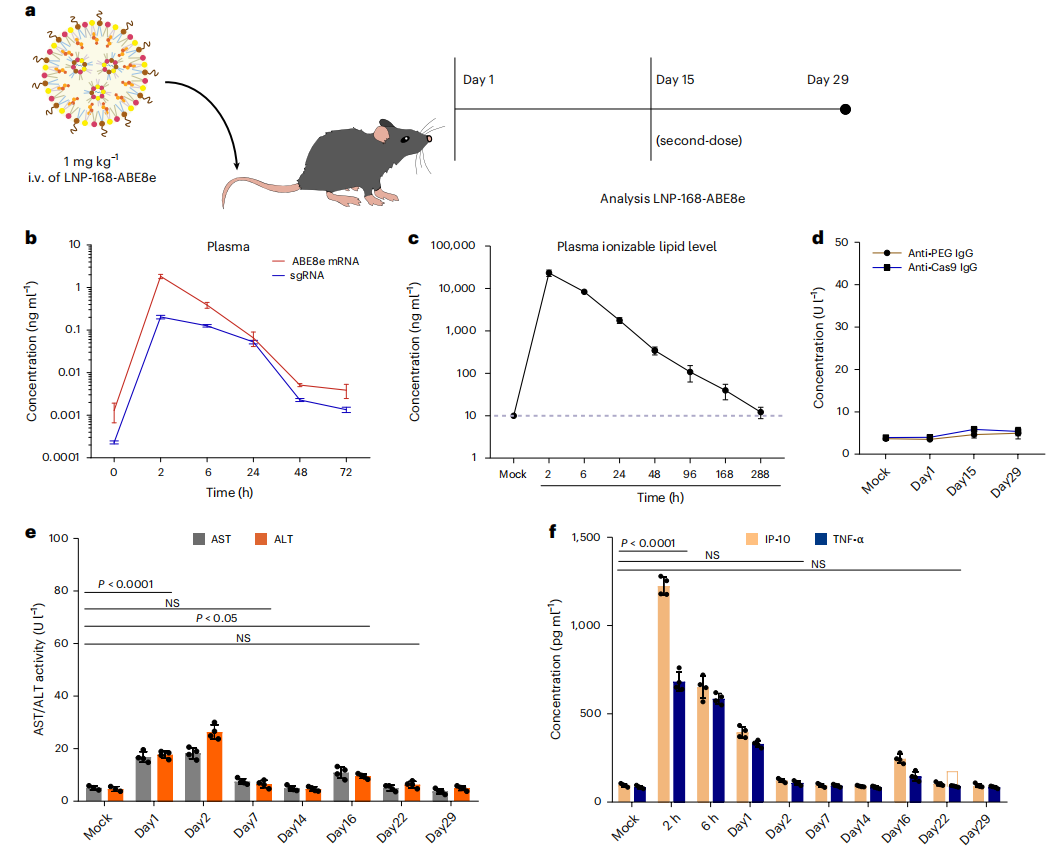
Beta-thalassemia and sickle cell disease are hereditary blood disorders caused by mutations in the hemoglobin gene, and patients typically require lifelong blood transfusions or hematopoietic stem cell transplantation. Current gene therapies primarily rely on ex vivo gene editing strategies, in which patients’ hematopoietic stem cells are extracted, genetically modified in the laboratory, and then reinfused. This approach is complex, costly, and requires chemotherapy to eliminate existing cells, resulting in significant side effects and limited applicability. Recently, a team of scientists from China, published a study in Nature Biomedical Engineering entitled “In vivo genome editing of human haematopoietic stem cells for treatment of blood disorders using mRNA delivery.” The study developed antibody-free lipid nanoparticles (LNP-168) capable of delivering genetic instructions (mRNA) directly to hematopoietic stem cells in the bone marrow, inducing reactivation of fetal hemoglobin expression and thereby restoring oxygen-carrying function in the blood. Original link: https://doi.org/10.1038/s41551-025-01480-y Spotlight 1. In vivo editing without stem cell extraction Antibody-free targeted lipid nanoparticles (LNPs) can deliver ABE8e mRNA and sgRNA directly to hematopoietic stem cells in the bone marrow via intravenous injection, enabling efficient gene editing without the need for stem cell extraction. 2. Efficient activation of fetal hemoglobin (HbF) 3. High safety profile and repeatable dosing The researchers first performed high-throughput sgRNA screening using HUDEP-2 cells (human erythroid progenitor cell line) expressing ABE8e. A total of 92 sgRNAs were designed to tile the promoter region of the γ-globin gene (HBG). Flow cytometry was then used to sort “HbF-high” and “HbF-low” cell populations, allowing identification of highly efficient sgRNAs, such as sgRNA-25. Figure 1. High-throughput sgRNA screening using the HUDEP-2 cell line The researchers designed three libraries of ionizable lipids (Library A/B/C), varying the head groups, linkers, and tail structures to identify lipids most suitable for bone marrow delivery, ultimately selecting LNP-168. Using a microfluidic mixing platform, they formulated LNPs encapsulating ABE8e mRNA and sgRNA—the “gene couriers.” The resulting mixed LNPs had particle sizes of approximately 70-90 nm, encapsulation efficiency greater than 90%, and good serum stability, providing a solid foundation for subsequent in vivo gene delivery. Figure 2. Screening and formulation of mRNA and sgRNA with LNP-168 The researchers transplanted CD34⁺ HSPCs from healthy donors into immunodeficient NCG-X mice to establish a humanized mouse model. Sixteen weeks later, LNP-168-ABE8e-HBG (LNPs encapsulating ABE8e mRNA and sgRNA-25) was administered via tail vein injection. The study demonstrated that these “gene couriers” could precisely target the bone marrow, edit human hematopoietic stem cells, and successfully activate γ-globin expression. Figure 3. Transplantation of CD34⁺ HSPCs carrying CD41-42 and β-28(A>T) mutations into NCG-X mice To evaluate the safety of this approach, the researchers collected plasma from experimental mice at various time points. Serum analysis of ALT/AST levels and inflammatory cytokines (IL-6, TNF, IP-10) showed no evidence of persistent liver injury or immune response. Next-generation sequencing (NGS) of 38 potential off-target sites revealed no significant off-target editing. Finally, by incorporating a gene delivery code miR-122T, editing activity in the liver was further reduced, ensuring that gene delivery was more specifically targeted to the bone marrow and enhancing overall targeting precision. Figure 4. Safety assessment via serum/plasma collection at different time points This study developed a non-viral, antibody-free LNP delivery system capable of efficient and safe in vivo editing of hematopoietic stem cells. This strategy bypasses traditional gene therapy procedures, including stem cell extraction, ex vivo culture, and chemotherapy preconditioning, significantly improving treatment accessibility and safety. In the future, patients may be able to achieve in vivo gene correction through a single intravenous administration, eliminating the need for painful chemotherapy and complex transplantation procedures, thereby substantially reducing the treatment burden.

By screening optimal sgRNAs, such as sgRNA-25, γ-globin expression was successfully reactivated in HSCs derived from β-thalassemia patients, restoring hemoglobin balance and correcting defects in red blood cell maturation.
The LNP system exhibits low immunogenicity, biodegradability, and suitability for repeated administration. No significant liver toxicity or immune responses were observed in experiments. Furthermore, the incorporation of a miR-122 target sequence further reduced off-target editing in the liver.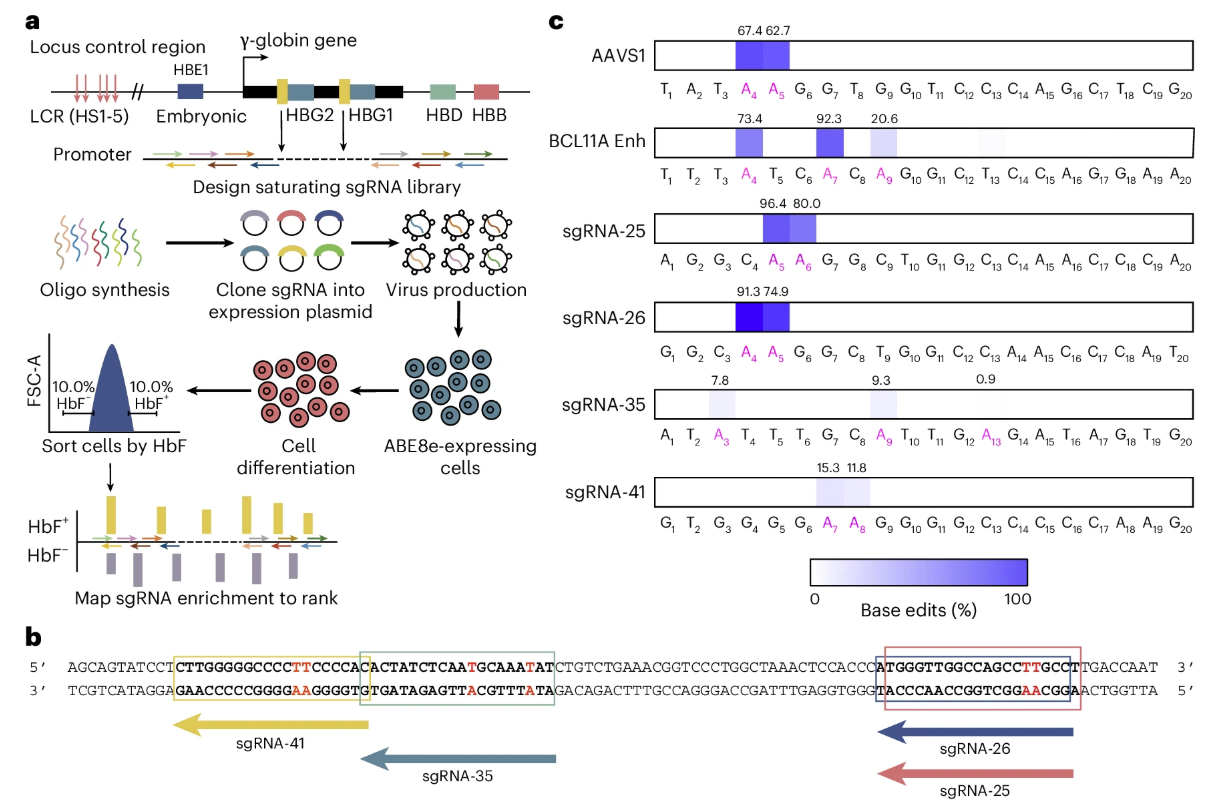













![[Literature Review] Bone Marrow–Targeted LNPs Open New Paths for Hematological Treatment](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)

Comment (4)