At Just One Year Old, He Joins Nature's 10: The Story of a CRISPR Breakthrough

The Youngest Entry in the History of "Nature’s 10"
He was not chosen for conducting research, but for being the subject of a medical milestone that redefined the boundaries of gene editing and therapy.
His name is KJ Muldoon.
Figure 1. KJ Muldoon (Source: Nature)
Previously, there have been pioneering cases in personalized gene therapy. The most well-known was Mila, whose treatment (Milasen) was custom-designed for a specific mutation in her genome. While it did not fully cure her, it successfully slowed the progression of her disease. This attempt laid the foundation for personalized medicine and is widely regarded as a milestone for N-of-1 (single-patient) gene therapy.
Following Mila, other patients have achieved significant results through gene editing, but most of those cases involved ex vivo editing (where cells are edited outside the body and then re-infused).
KJ’s treatment, however, represents a radical leap: it is the world’s first in vivo personalized CRISPR therapy. Scientists encapsulated the gene-editing tools within lipid nanoparticles (LNPs) and injected them directly into KJ’s bloodstream. These particles specifically targeted liver cells to perform DNA repair. Theoretically, this means KJ may only require a limited number of treatments to achieve a permanent cure.
Just 48 hours after birth, KJ began exhibiting symptoms of lethargy and respiratory distress.
Blood tests revealed a staggering blood ammonia concentration of 1,703 μmol/L—more than 50 times the normal range. Such levels are typically seen only in severe urea cycle disorders or acute liver failure.
In healthy individuals, blood ammonia levels range from 9-33 μmol/L; as ammonia accumulates, it causes neurological damage, including lethargy and seizures. Further elevation leads to coma, cerebral edema, and death.
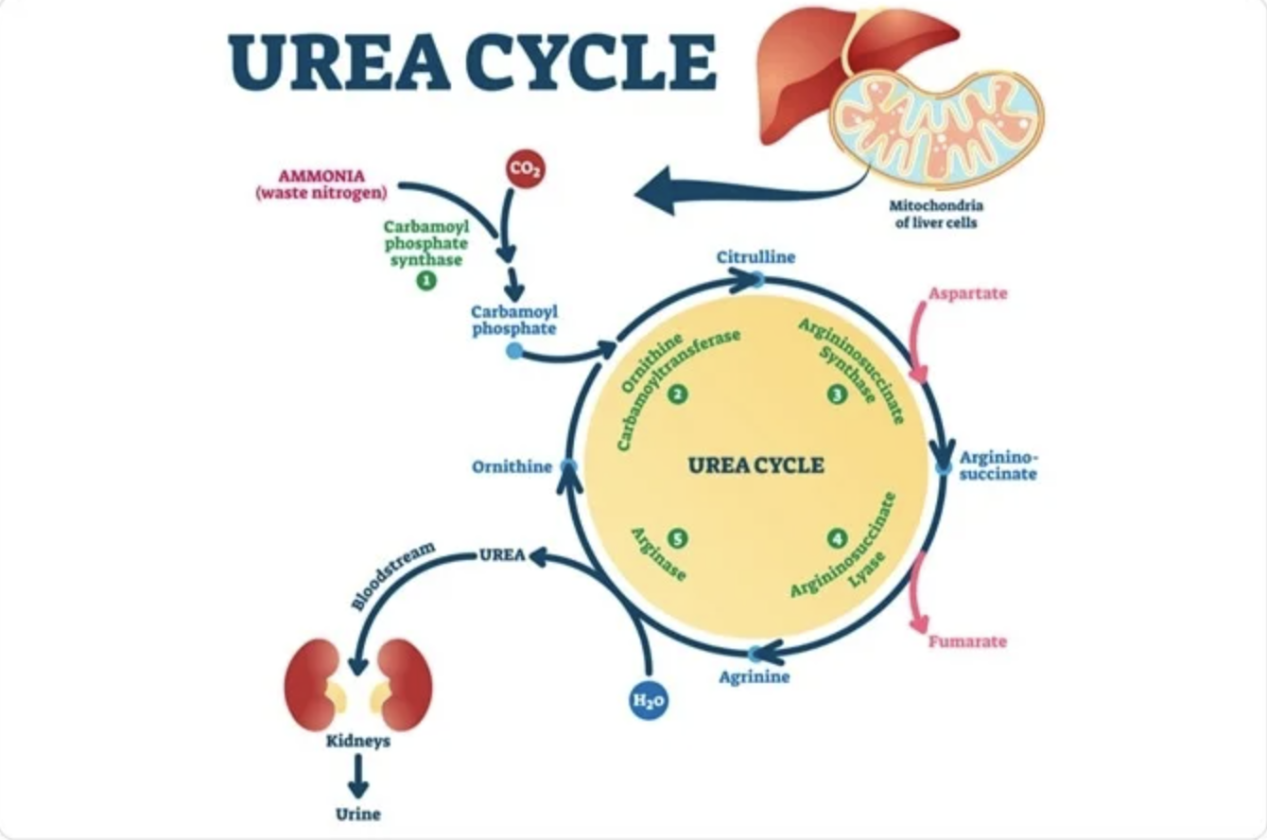
Figure 2. Schematic of the urea cycle
The urea cycle is a critical metabolic pathway in the liver. Its primary function is to convert toxic ammonia (NH₃), a byproduct of protein breakdown, into non-toxic urea, which is then excreted via the kidneys. This process safely removes excess nitrogen and maintains nitrogen balance.
The results showed two nonsense mutations in KJ’s CPS1 gene: c.1003C→T and c.2140G→T, inherited from each parent. KJ was formally diagnosed with Carbamoyl Phosphate Synthetase 1 (CPS1) deficiency. This is an extremely rare genetic disorder with an estimated incidence of less than one in a million. Although individual rare diseases are scarce, they collectively affect millions worldwide, often with high mortality rates and little hope for survival.
Conventional treatments, such as low-protein diets and liver transplantation, were not viable options for KJ at the time, as his infant body could not withstand the high risks of a transplant surgery.
Faced with KJ’s deteriorating condition, pediatricians and scientists collaborated to attempt a tailor-made solution using cutting-edge CRISPR technology.
The research team utilized Base Editing, a derivative of CRISPR genome editing. This technology allows for the precise correction of a mutation by swapping a single DNA base pair. By avoiding the double-strand breaks used in traditional CRISPR, it minimizes the risk of indel errors and reduces off-target effects.

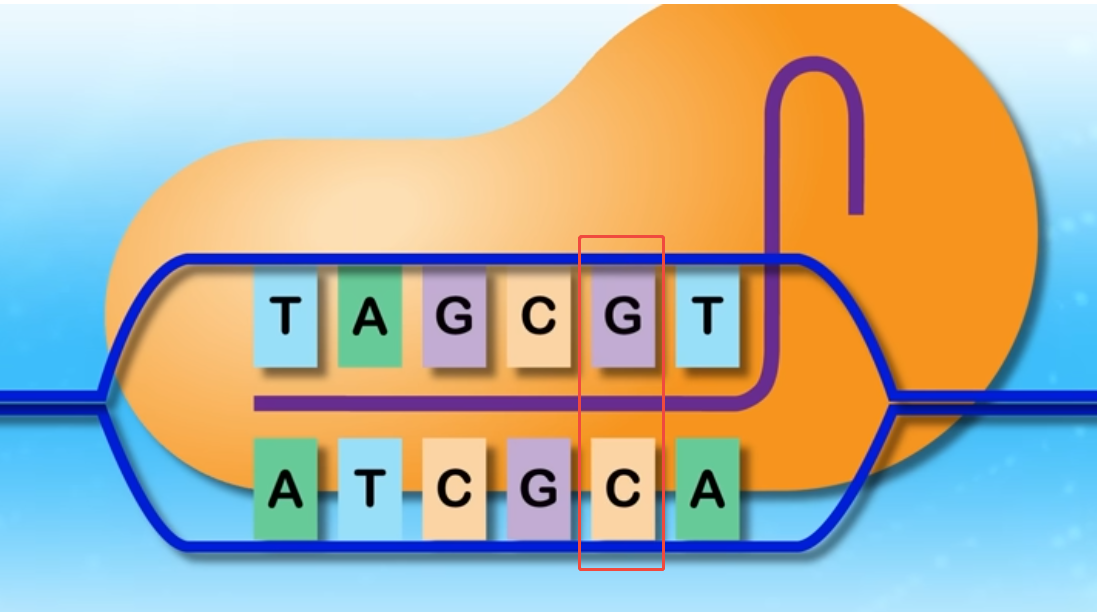
Figure 3. Base Editing
The team targeted the Q335X mutation (c.1003C→T) inherited from KJ’s father. Using an Adenine Base Editor (ABE), they targeted the A-T base pair at the mutation site and converted it into a normal G-C base pair. This restored the correct amino acid sequence and, consequently, the function of the CPS1 gene.
To ensure the editing tools reached the liver accurately, researchers used lipid nanoparticles (LNPs) as delivery vehicles. The CRISPR editors were encapsulated within these LNPs and administered to KJ via intravenous injection.
This highly customized therapy, named "k-abe," became the world’s first personalized CRISPR gene-editing therapy successfully applied to an infant.
• At ~1 month old: A patient-specific cell line was established to study KJ’s unique genetic sequence.
• At ~6 months old: As his condition worsened, KJ was officially placed on the liver transplant waiting list.
Simultaneously:
• Researchers worked around the clock to manufacture the gene-editing components in just six months—a process that normally takes 18 months.
• The team conducted comprehensive testing, including toxicology studies in non-human primates and mice.
• The team submitted a “Compassionate Use” application to the FDA; it was approved within a week, allowing the treatment to proceed.
On February 25, 2025—209 days after birth—KJ received the world’s first personalized in vivo CRISPR treatment. The LNPs entered his circulation, were taken up by hepatocytes, and successfully repaired the pathogenic mutation in the CPS1 gene.
Since then, KJ’s blood ammonia levels have not seen a significant spike. He has been able to gradually increase his protein intake, and his vital signs remain stable. While it is too early to declare a "total cure," the marked improvement in the early stages of treatment has brought new hope for his future.
As of April 2025, KJ has received three doses of the therapy with no serious side effects. Shortly after treatment, he began tolerating increased protein intake and required fewer nitrogen scavengers.
In May 2025, a report from the National Institutes of Health (NIH) referred to KJ as the "first infant to successfully receive personalized gene therapy." His treatment was meticulously designed to target non-germline cells, ensuring the genetic changes would not be passed to future generations.
Figure 4. NIH related report
KJ’s success has sparked widespread interest in the medical community regarding personalized gene therapy.
His story is more than a personal victory over a life-threatening illness; it represents a massive leap in the application of genome engineering. It showcases the vast potential of CRISPR in personalized medicine, especially for addressing various rare genetic diseases.
Through personalized therapy, CRISPR has evolved from a laboratory concept into a viable clinical solution. The U.S. FDA recently announced an accelerated approval pathway for personalized treatments for rare genetic diseases, opening new doors for clinical applications.
Challenges remain, including long-term safety, the stability of therapeutic effects, and the high cost of customized treatments. Is KJ’s "N=1" miracle a prologue to the widespread adoption of gene therapy, or a unique case difficult to replicate?
The answer will be written by time and continued scientific endeavor.
1.Ledford, H. (2025). World’s first personalized CRISPR therapy given to baby with genetic disease. Nature, News Feature.
2.Ledford, H. (2025). The baby whose life was saved by the first personalized CRISPR therapy. Nature, News Feature.
3.National Center for Advancing Translational Sciences (NCATS). (2025). Infant with rare, incurable disease is first to successfully receive personalized gene therapy treatment. National Institutes of Health (NIH).

















