[Literature Review] How a Single Exercise Session Rapidly Alleviate Depression?
Knockdown Cell Line
Gene Editing

For decades, the treatment of depression has relied mainly on medication, which often requires long-term use and shows limited efficacy. Although clinical studies have shown that even a single session of exercise can rapidly alleviate depressive symptoms, the underlying biological mechanisms have remained unclear.
Recently, a research team led by Chinese scientists,published a study in Molecular Psychiatry titled “Rapid antidepressant effect of single-bout exercise is mediated by adiponectin-induced APPL1 nucleus translocation in anterior cingulate cortex.” The research unveiled the key signaling pathway behind this rapid antidepressant effect.
Using C57BL/6J wild-type mice and Adiponectin knockout models, the team combined AAV vector-mediated delivery of shRNA in the anterior cingulate cortex (ACC) to achieve neuron-specific knockdown of APPL1 or AdipoR1. Their findings revealed that adiponectin—a hormone secreted by peripheral adipose tissue—acts as the critical messenger that initiates rapid antidepressant responses in the brain, offering a novel target for developing fast-acting antidepressant therapies.
Original link: https://doi.org/10.1038/s41380-025-03317-1
Spotlights
1. First to reveal the role of the adipose–brain axis in rapid antidepressant effects:
The study identified and validated adiponectin as a peripheral adipokine capable of crossing the blood–brain barrier and acting on glutamatergic neurons in the anterior cingulate cortex (ACC), mediating the rapid antidepressant effects of exercise.
2. Discovery of a novel function of APPL1 nuclear translocation:
Beyond serving as an adiponectin signaling adaptor, APPL1’s nuclear accumulation was found to regulate synaptic plasticity through epigenetic mechanisms, unveiling a new pathway underlying fast-acting antidepressant responses.
3. Identification of potential therapeutic targets:
The study highlights AdipoR1 and APPL1 nuclear translocation as promising targets for developing rapid-onset antidepressant drugs.
The researchers first conducted a 30-minute running exercise with human participants, both with and without depressive symptoms, and observed a clear reduction in negative emotions after exercise.
Similarly, in mice subjected to chronic unpredictable mild stress (CUMS) or control conditions, a 30-minute treadmill exercise produced rapid and lasting antidepressant-like behavioral effects, as confirmed through a series of behavioral tests conducted at different time points post-exercise.
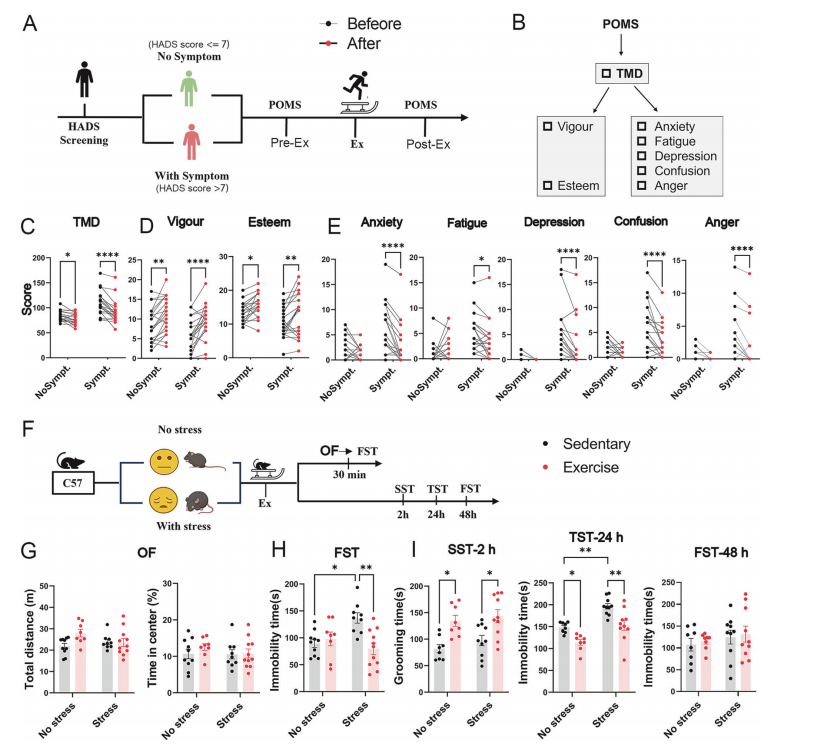
Figure 1. Validation of the antidepressant effect of exercise from clinical subjects to mouse models
To determine which brain regions and cell populations are activated by a single bout of exercise, the researchers performed immunofluorescence staining for c-Fos and neuronal subtype markers across brain sections.
The results pinpointed glutamatergic neurons (CaMKII⁺) in the anterior cingulate cortex (ACC) as the primary cells activated after exercise. In vivo calcium imaging further confirmed a significant increase in the activity of these ACC neurons immediately following exercise.
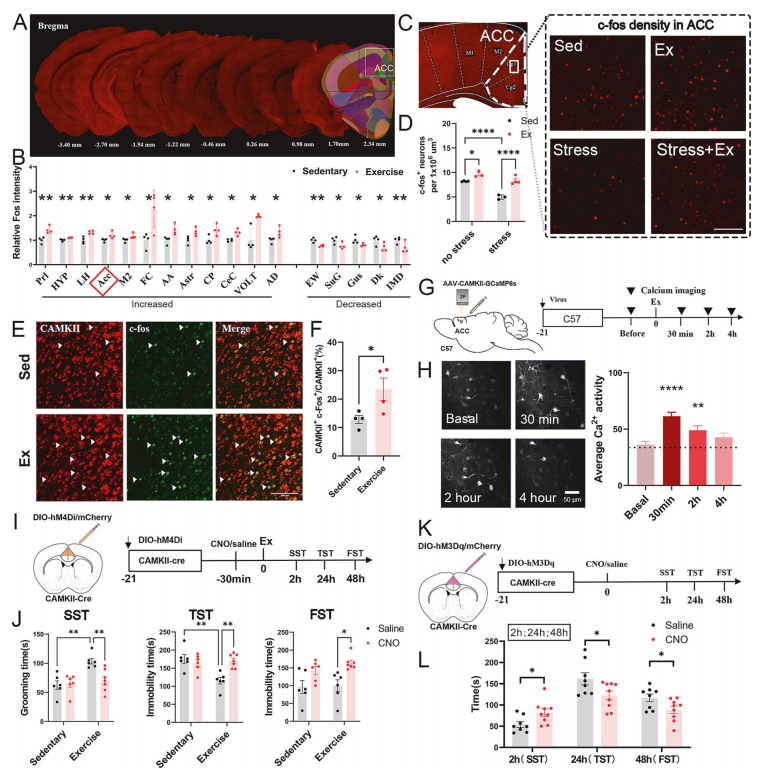
Figure 2. Causal link between ACC CaMKII⁺ neuron activation and antidepressant effects
To uncover the peripheral signaling underlying the antidepressant response to exercise, the researchers measured Adiponectin levels using ELISA and found that a single bout of exercise significantly increased Adiponectin in the medial prefrontal cortex (mPFC), alleviating depressive behaviors.
Further investigation revealed that this effect was mediated through AdipoR1 receptors expressed on ACC glutamatergic neurons. Genetic validation confirmed this pathway’s necessity: in global Adiponectin knockout mice or neuron-specific AdipoR1 knockdown models, both exercise-induced neuronal activation and the resulting antidepressant behaviors were abolished.
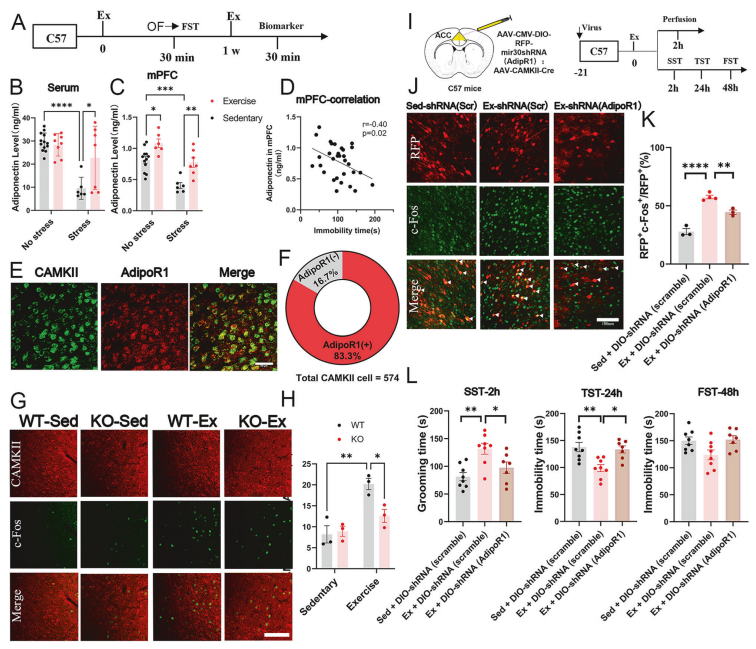
Figure 3. Confirmation that adiponectin acts on ACC CaMKII⁺ neurons via its receptor adipoR1
Through gain- and loss-of-function experiments, the researchers demonstrated that APPL1 plays a pivotal role in the antidepressant effect induced by exercise. Neuron-specific knockdown of APPL1 in the ACC completely abolished the antidepressant response, while re-expression of APPL1 in global APPL1 knockout mice successfully rescued the phenotype.
Mechanistically, exercise did not alter APPL1 mRNA levels, indicating that regulation occurs at the protein level. Immunofluorescence quantification further confirmed that APPL1 translocates from the cytoplasm into the nucleus following exercise. Importantly, this nuclear translocation was absent in Adiponectin knockout or AdipoR1 knockdown mice, establishing that APPL1 translocation depends on upstream Adiponectin-AdipoR1 signaling.
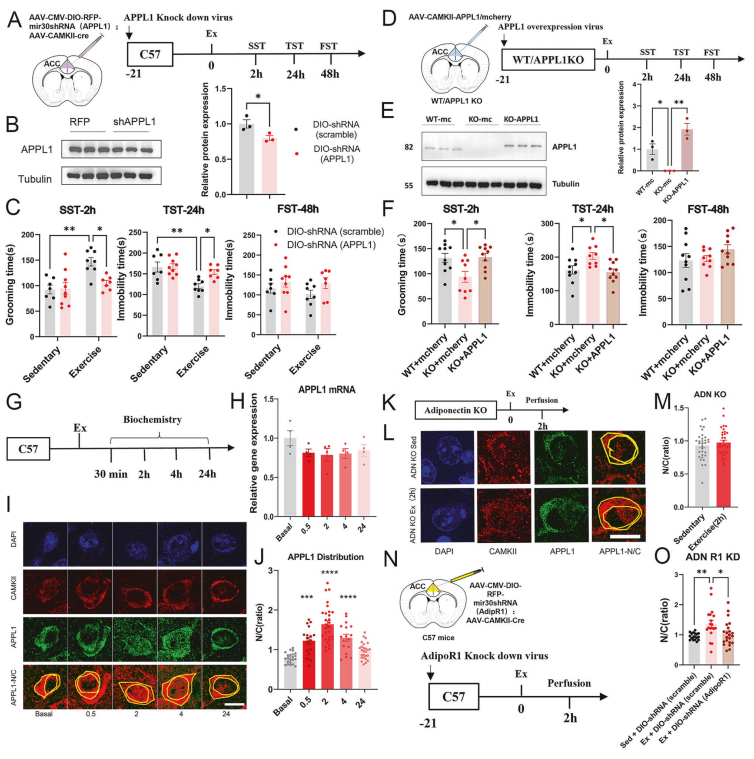
Figure 4. Downstream adapter protein APPL1 translocates into the nucleus following adipoR1 activation
The researchers proposed a molecular model illustrating how APPL1 nuclear translocation mediates the antidepressant effect of exercise. Western blot analysis confirmed that a single bout of exercise induced histone H4 acetylation through APPL1 nuclear localization, leading to sustained upregulation of synaptic proteins and the formation of new dendritic spines.
Moreover, pharmacological inhibition of APPL1 nuclear translocation completely blocked these effects—histone acetylation, synaptic protein elevation, dendritic spine formation, and the behavioral antidepressant response—demonstrating that APPL1 acts as a critical epigenetic regulator linking peripheral signals to synaptic remodeling and mood improvement.
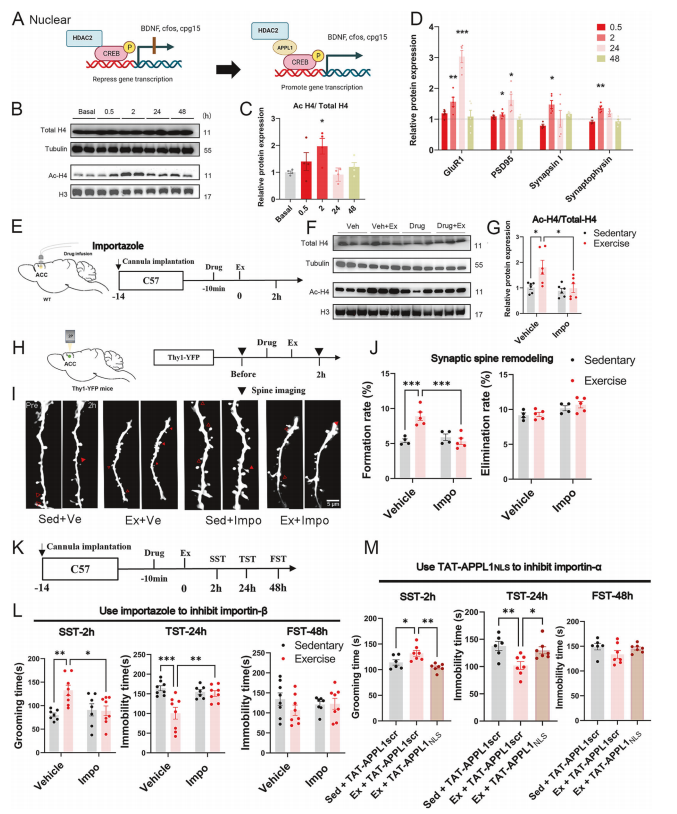
Figure 5. Exercise-induced changes in microglial state distribution in female mice
This study uncovers the core mechanism of the “exercise-adipose-brain” axis in rapidly alleviating depressive symptoms, identifying the AdipoR1 receptor and its downstream APPL1 nuclear translocation as promising therapeutic targets for the development of fast-acting antidepressants.
These findings not only provide a scientific explanation for how a single session of exercise can swiftly elevate mood, but also establish a solid foundation for considering exercise as a viable non-pharmacological strategy for rapid relief of depressive symptoms.
At EDITGENE, our custom gene knockout cell line services utilize an advanced CRISPR/Cas9 system to support research teams in bridging basic research and clinical applications. Feel free to reach out anytime to design a gene editing plan tailored to your research needs.
Recent Blogs
1. [Practical Insights]Designing Donor Templates
2. [Literature Insight] The Misjudgment of Microglia: How TREM2 Mutations Disrupt Brain Balance
3. [Quality Share]A Comprehensive Guide to CRISPR Detection
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com









![[Literature Review] How a Single Exercise Session Rapidly Alleviate Depression?](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)

Comment (4)