[Literature Review] What Causes Abnormal Sperm Morphology? ZDHHC19 Unveils the Chromatin Remodeling Code
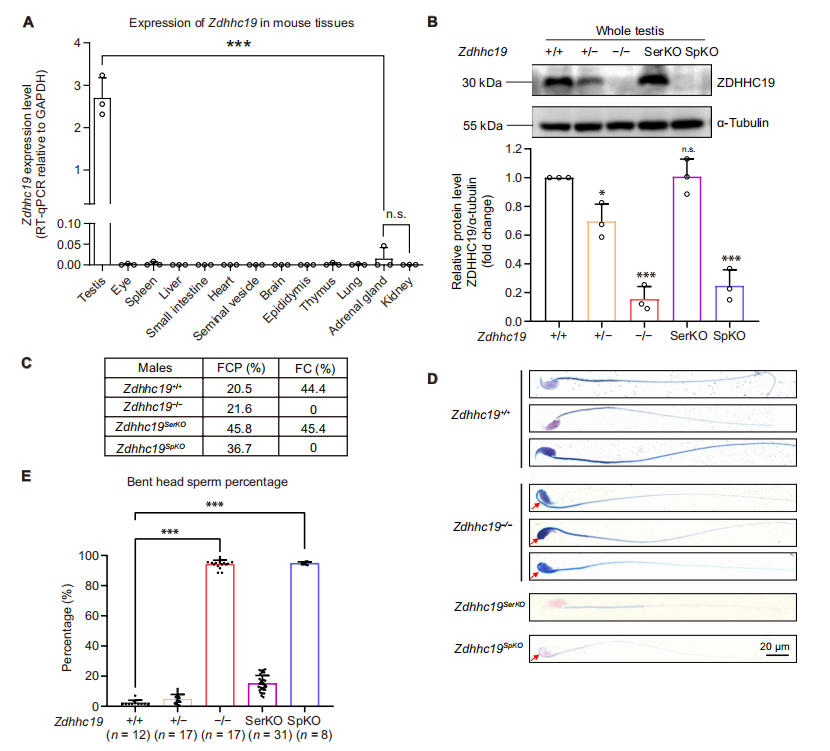
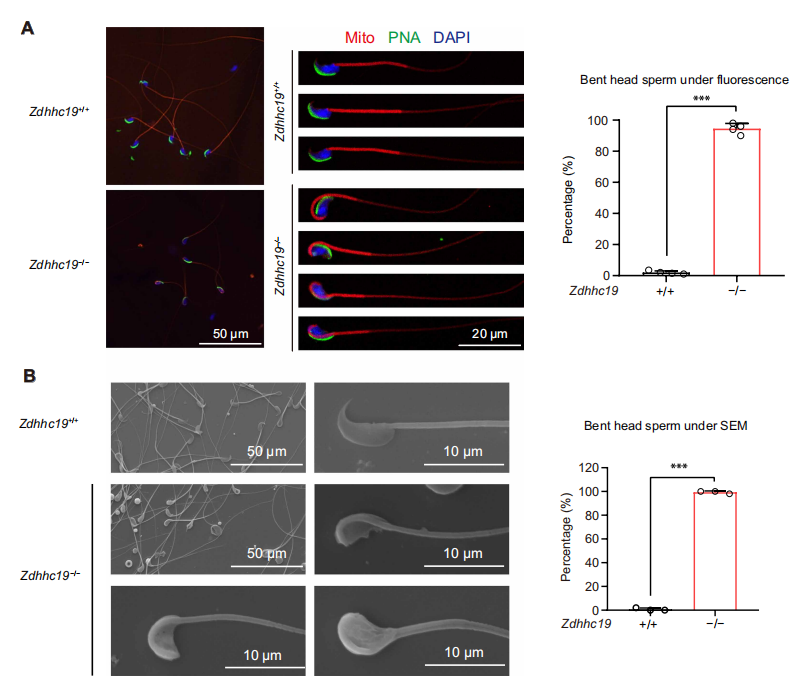
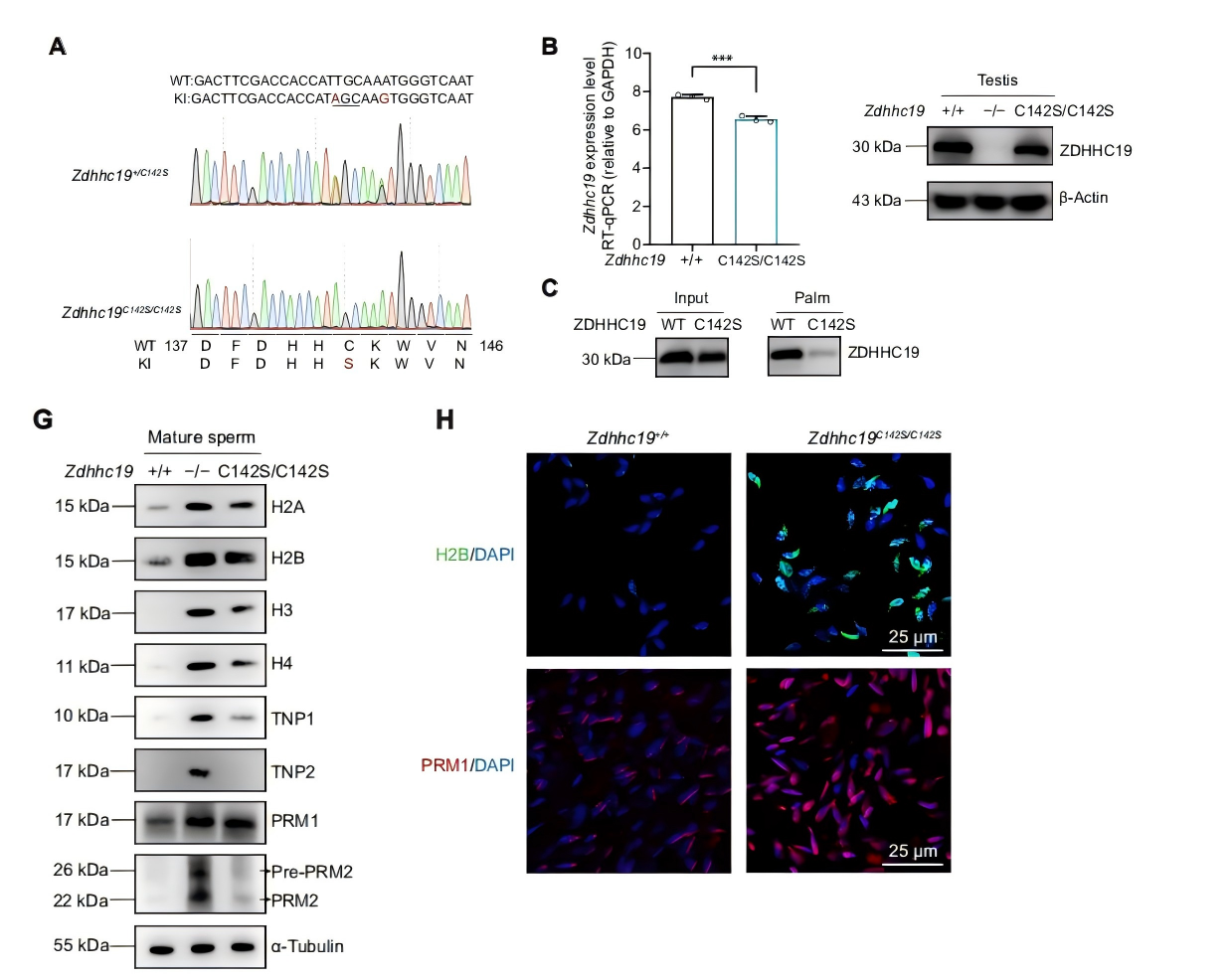
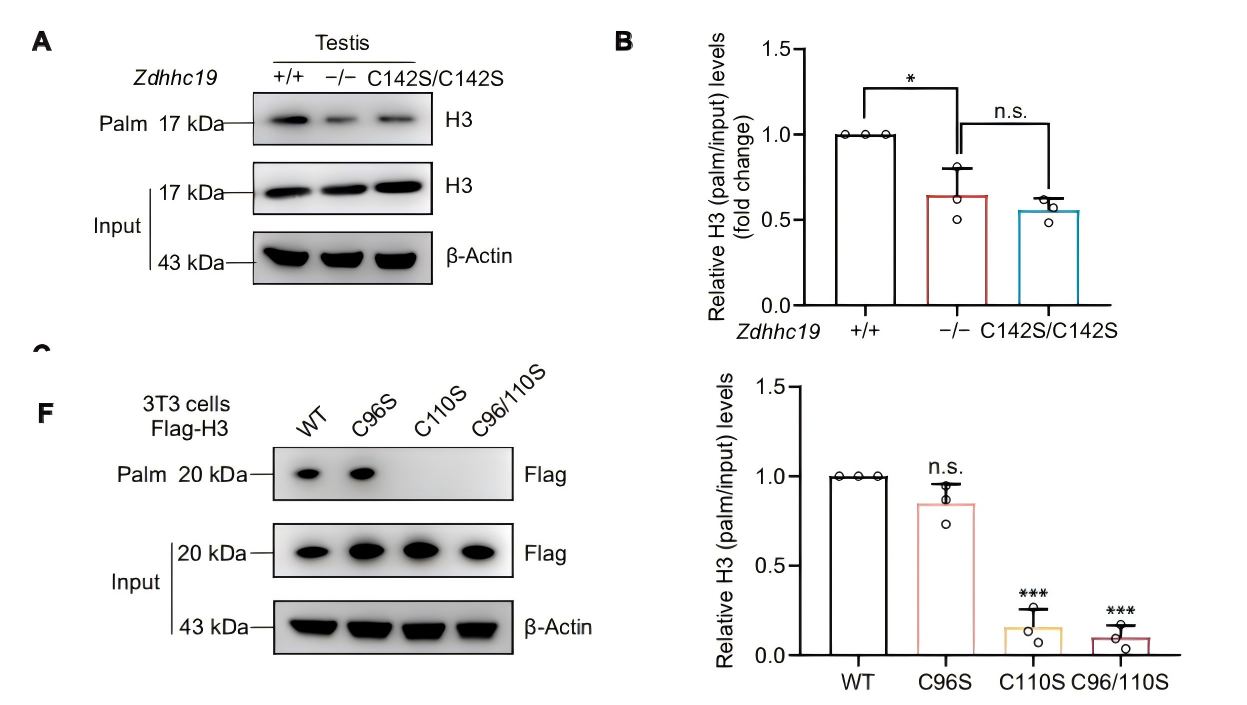
Spermatogenesis is a highly orchestrated differentiation process. One of its most crucial steps is the histone-to-protamine exchange, during which histones are replaced by protamines to achieve the extreme chromatin compaction required for mature sperm formation. Disruption of this process often results in loosely packed chromatin, impaired sperm motility, and even male infertility. ZDHHC19, a palmitoyltransferase containing a conserved DHHC domain, has previously been shown to regulate protein localization and function in tumors, the nervous system, and the immune system. However, its role in reproduction remained largely unexplored. On October 17, 2025, a joint research team from China published a study in Science Advances titled “Palmitoyltransferase ZDHHC19 regulates histone-to-protamine exchange during spermiogenesis in mice.” This study is the first to demonstrate that ZDHHC19 catalyzes the palmitoylation of histone H3 at cysteine 110, thereby regulating the critical histone-to-protamine exchange during spermiogenesis. These findings provide new mechanistic insights into chromatin remodeling in sperm development and offer potential therapeutic targets for male infertility. Original link: https://www.science.org/doi/10.1126/sciadv.adv5189 Spotlight 1.Technical Breakthrough: Precise Multi-Model Construction to Elucidate Zdhhc19 Function The research team employed CRISPR/Cas9 technology to generate three mouse models — global knockout, cell type–specific knockout, and catalytic site point mutant (C142S) — providing a systematic understanding of the origin and mechanism of Zdhhc19 function. 2.Novel Mechanistic Insight: H3 Palmitoylation Regulates Chromatin Remodeling The study identified ZDHHC19 as the key enzyme catalyzing histone H3 palmitoylation at Cys110. This modification weakens the H3-H4 interaction and increases chromatin accessibility, thereby facilitating histone removal and protamine replacement during spermiogenesis. 3.Key Discovery: ZDHHC19 Deficiency Leads Directly to Male Infertility Male mice lacking Zdhhc19 exhibited complete infertility, characterized by “bent-head” sperm morphology, insufficient chromatin condensation, and abnormal histone retention, uncovering a novel molecular mechanism underlying male infertility. Zdhhc19 is predominantly expressed in the testes of mice, with minimal or negligible expression in other tissues, and is highly enriched in haploid spermatids. The researchers first generated conditional knockout mouse models and found that male mice exhibited complete infertility and severe sperm deformities only when Zdhhc19 was deleted in germ cells, whereas knockout in supporting cells had no impact on fertility. Figure 1. Validation of germ Cell Function in Zdhhc19 Knockout Mice The study found that Zdhhc19 deletion does not affect overall testis morphology or the early stages of spermatogenesis. Further analyses revealed that Zdhhc19⁻/⁻ sperm frequently exhibited a 180° bend between the head and midpiece, a characteristic head-neck bending defect associated with impaired chromatin condensation. Electron microscopy and chromatin structural analyses showed that sperm nuclei lacking Zdhhc19 had reduced chromatin density, increased vacuolization, and a significantly higher rate of DNA fragmentation. Figure 2.Zdhhc19 deletion impairs spermatogenesis by disrupting chromatin condensation To determine whether the palmitoyltransferase (PAT) activity of ZDHHC19 is essential for spermatogenesis, the researchers generated Zdhhc19^C142S/C142S knock-in mice carrying a catalytic site mutation. Although this mutation did not markedly affect overall Zdhhc19 mRNA or protein levels, it significantly reduced ZDHHC19 auto-palmitoylation, confirming impaired PAT activity. Functional analyses showed that Zdhhc19^C142S/C142S male mice exhibited substantially reduced fertility, including lower pregnancy rates and significantly fewer pups per litter. Figure 3. Investigation of ZDHHC19 PAT activity in spermatogenesis Using palmitoylated proteome profiling, the research team identified histone H3 as a primary substrate of ZDHHC19. In vitro experiments demonstrated that H3 palmitoylation weakens H3-H4 interactions, facilitating nucleosome disassembly. ChIP-qPCR and ATAC-seq analyses revealed that in haploid spermatids of Zdhhc19⁻/⁻ mice, chromatin accessibility is reduced and H3–DNA binding is enhanced, thereby impeding protamine incorporation. Figure 4. Mass spectrometry and biochemical assays confirm Histone H3 as a direct substrate of ZDHHC19 This study demonstrates that palmitoylation, as a novel form of histone modification, regulates the critical histone-to-protamine exchange step, thereby orchestrating chromatin remodeling during spermatogenesis. This finding directly links a lipid modification mechanism to sperm chromatin remodeling for the first time, providing new insights into the molecular basis of male infertility.